A major focus of the Sperling lab is studying the mechanism of action of protein degraders. These drugs work by a unique mechanism in that they act as a molecular bridge or glue to bring together proteins or macromolecular complexes that would not otherwise interact (Image: Molecular glue.png). The discovery of drugs that work as molecular glues has enabled therapeutic targeting of non-enzyme proteins such as transcription factors and other difficult to drug proteins.
Thalidomide Analogs
Multiple myeloma (MM) is a plasma cell neoplasm with an incidence of over 20,000 new cases in the United States per year. While it is incurable, life expectancy has improved dramatically over the past two decades, in large part due to the development of a number of new drugs including thalidomide and its analogs lenalidomide and pomalidomide. Some of these agents are also active and approved in several other hematologic malignancies including non-Hodgkin lymphoma (NHL) and myelodysplastic syndrome (MDS).
Only recently has the mechanism by which thalidomide analogs exert their antineoplastic effect been described. By acting as a molecular glue and bringing target proteins and the E3 ubiquitin ligase CUL4-RBX1-DDB1-CRBN (known as CRL4CRBN) into proximity, thalidomide analogs mediate poly-ubiquitination and subsequent degradation of critical oncoproteins by the proteasome.
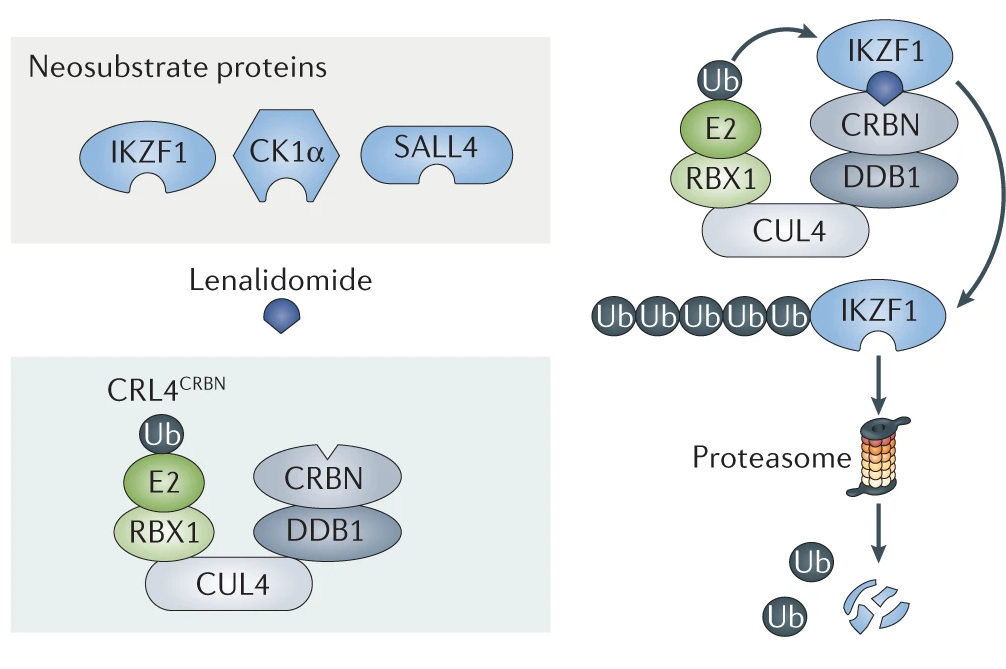
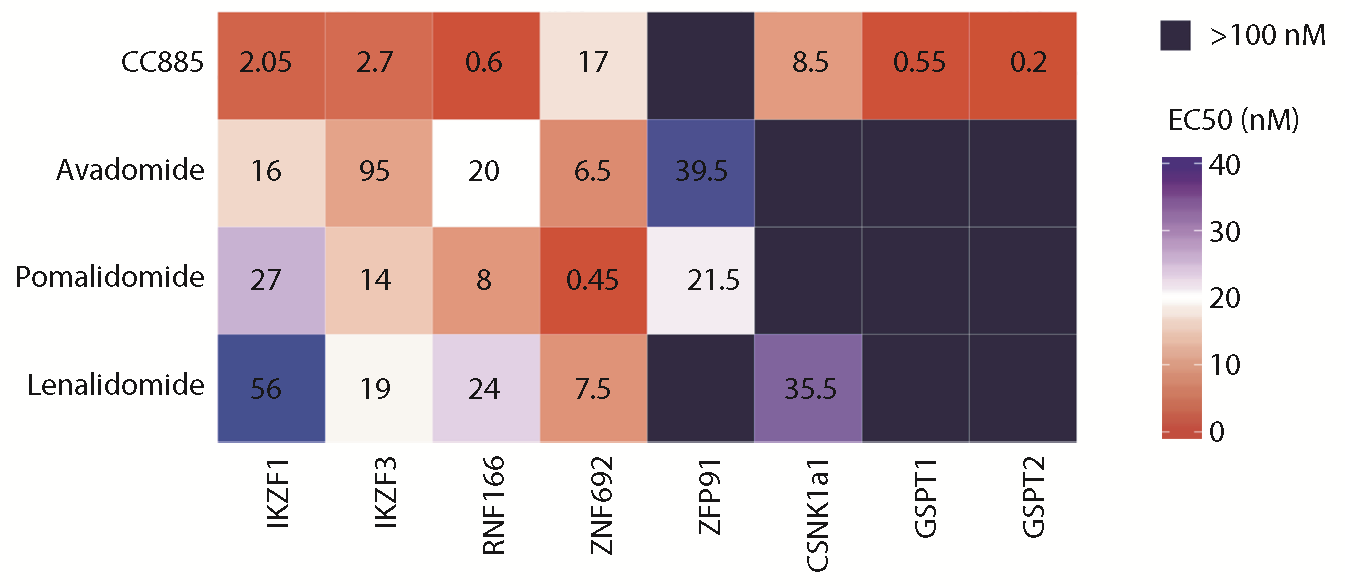
In MM and NHL, degradation of the core lymphocyte transcription factors IKZF1 and IKZF3 leads to growth arrest and eventual apoptosis. In del(5q) MDS, degradation of the haplo-insufficient factor CK1a, encoded by the CSNK1A1 gene on the common deleted region of 5q, is responsible for clinical activity. Different thalidomide analogs, such as pomalidomide and avadomide have different substrate specificity, and thus clinical activity.
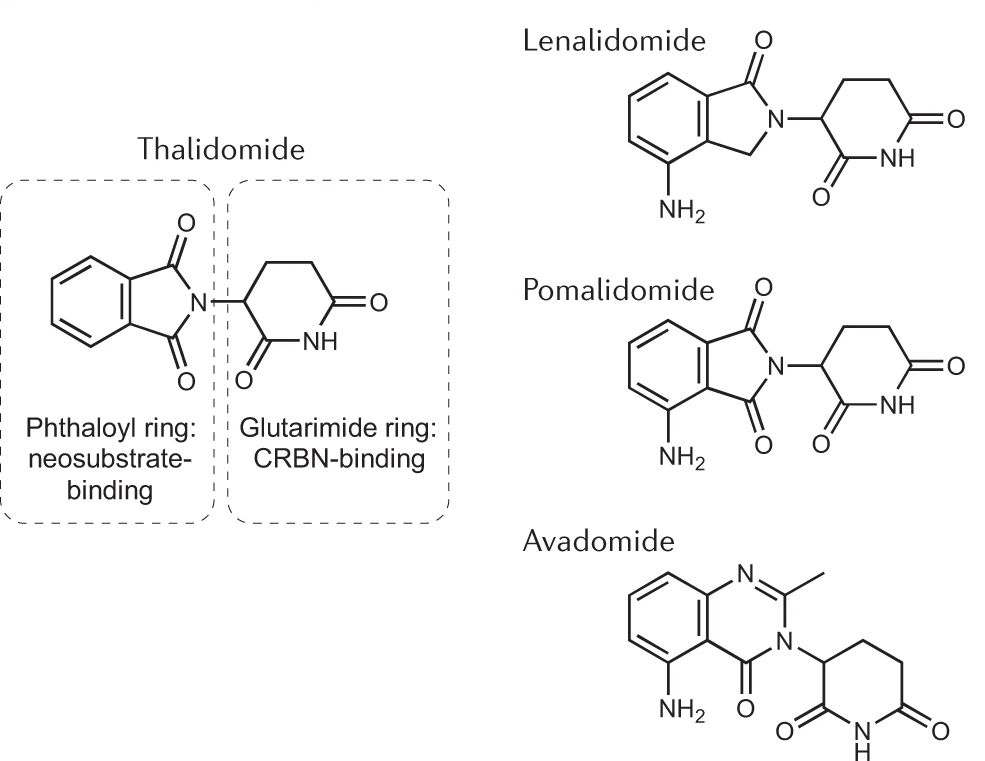
For instance, pomalidomide does not degrade CK1a in vitro and concomitantly has no activity in del(5q) MDS, highlighting the direct and specific relationship between target protein degradation and clinical activity.
MM patients who become refractory to all thalidomide analogs have a dismal prognosis making this an area of unmet clinical need. However, it is largely unknown what mediates resistance to these agents in patients or how to overcome it. A primary focus of the Sperling research lab is to determine the mechanisms of thalidomide analog activity to better understand how resistance develops and to identify new therapeutic targets.
Publications
- Degradation of GSPT1 causes TP53-independent cell death in leukemia whilst sparing normal hematopoietic stem cells. Sellar RS*, Sperling AS*, Słabicki M, Gasser JA, McConkey ME, Donovan KA, Mageed N, Adams DN, Zou C, Miller PG, Dutta RK, Boettcher S, Lin AE, Sandoval BE, Quevedo Barrios VA, Shkolnik V, Koeppel J, Henderson EK, Fink EC, Yang L, Chan AK, Pokharel SP, Bergstrom EJ, Burt R, Udeshi ND, Carr SA, Fischer ES, Chen CW, Ebert BL. J Clin Invest. 2022 Aug 15;132(16):e153514. doi: 10.1172/JCI153514.
- Cancer therapies based on targeted protein degradation – lessons learned with lenalidomide. Jan M, Sperling AS and Ebert BL. Nat Rev Clin Oncol. 2021 Jul;18(7):401-17. doi: 10.1038/s41571-021-00479-z.
- Small-molecule-induced polymerization triggers degradation of BCL6. Słabicki M, Yoon H, Koeppel J, Nitsch L, Roy Burman SS, Di Genua C, Donovan KA, Sperling AS, Hunkeler M, Tsai JM, Sharma R, Guirguis A, Zou C, Chudasama P, Gasser JA, Miller PG, Scholl C, Fröhling S, Nowak RP, Fischer ES, Ebert BL. Nature. 2020 Dec;588(7836):164-168.
- The CDK inhibitor CR8 acts as a molecular glue degrader that depletes cyclin K. Słabicki M, Kozicka Z, Petzold G, Li YD, Manojkumar M, Bunker RD, Donovan KA, Sievers QL, Koeppel J, Suchyta D, Sperling AS, Fink EC, Gasser JA, Wang LR, Corsello SM, Sellar RS, Jan M, Gillingham D, Scholl C, Fröhling S, Golub TR, Fischer ES, Thomä NH, Ebert BL. Nature. 2020 Sep;585(7824):293-7. doi: 10.1038/s41586-020-2374-x
- Patterns of substrate affinity, competition, and degradation kinetics underlie biological activity of thalidomide analogs. Sperling AS, Burgess M, Keshishian H, Gasser JA, Bhatt S, Jan M, Słabicki M, Sellar RS, Fink EC, Miller PG, Liddicoat BJ, Sievers QL, Sharma R, Adams DN, Olesinski EA, Fulciniti M, Udeshi ND, Kuhn E, Letai A, Munshi NC, Carr SA, Ebert BL. Blood. 2019 Jul 11;134(2): 160-170.
- A phase I study of lenalidomide plus chemotherapy with mitoxantrone, etoposide, and cytarabine for the reinduction of patients with acute myeloid leukemia. DeAngelo DJ, Brunner AM, Werner L, Avigan D, Fathi AT, Sperling AS, Washington A, Stroopinsky D, Rosenblatt J, McMasters M, Luptakova K, Wadleigh M, Steensma DP, Hobbs GS, Attar EC, Amrein PC, Ebert BL, Stone RM, Ballen KK. Am J Hematol. 2018 Feb;93(2):254-61. doi: 10.1002/ajh.24968.