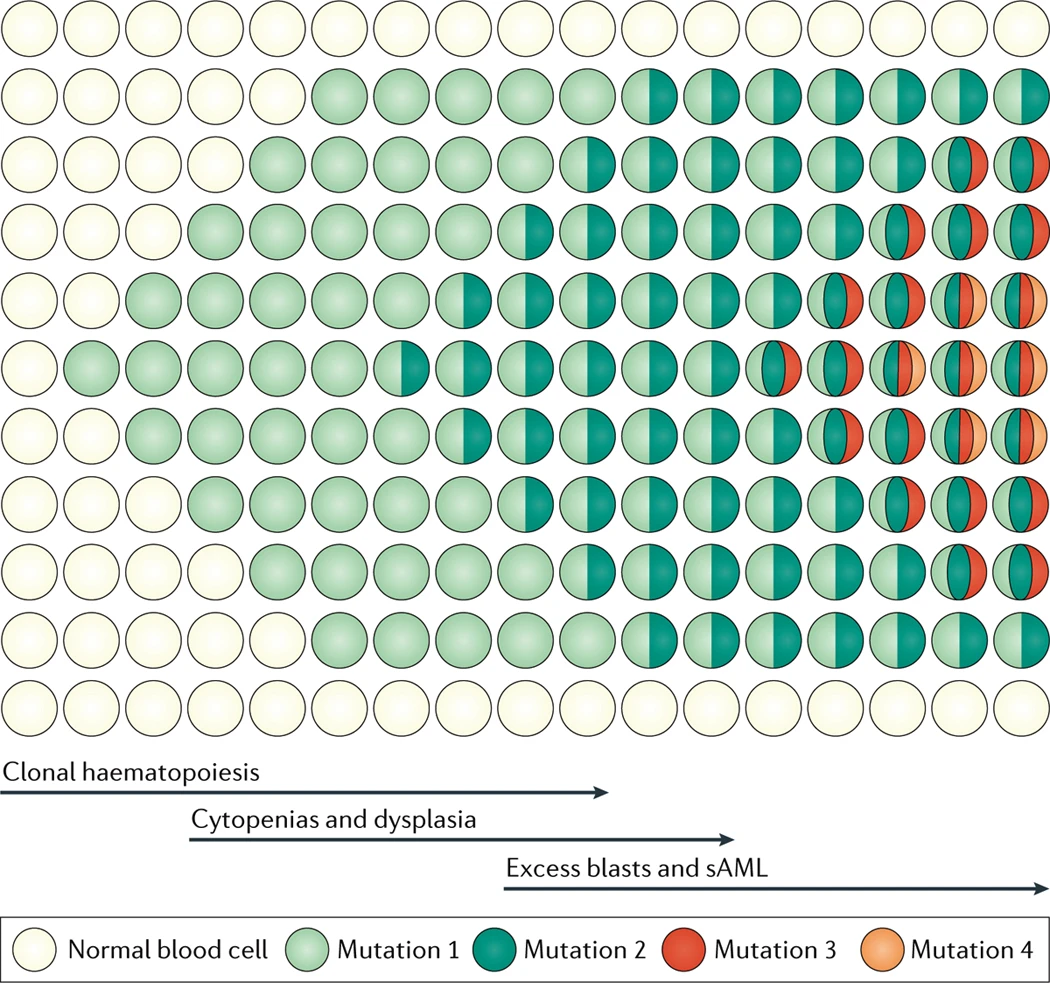
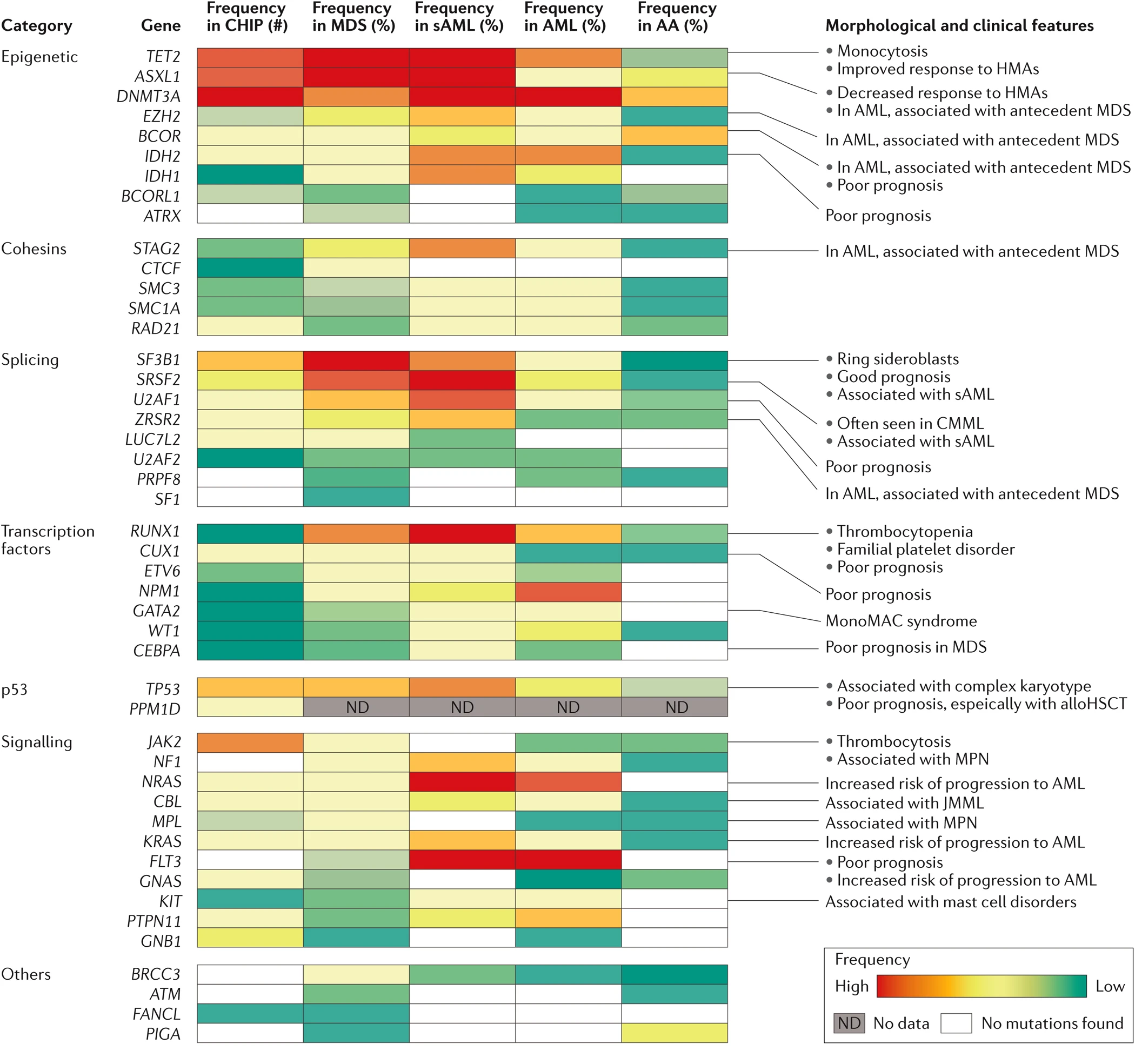
Hematopoietic stem cells (HSCs) can acquire somatic mutations that promote clonal expansion, an age-related process detectable in the blood normal individuals. This process is associated with an increased risk of developing hematologic malignancies and with a number of inflammatory disorders including cardiovascular disease, COPD and rheumatologic disease.
Therapy Related Myeloid Neoplasms
Therapy related myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML), collectively referred to as therapy related myeloid neoplasms (tMN), are blood cancers that arise following the administration of chemotherapy and radiation, usually given for other solid tumors or lymphomas. tMNs are among the most devastating complications of cancer therapy, with a median overall survival (OS) of 7-10 months and a 5-year OS of 10-20%. The old model of tMN development in which mutations accumulate in hematopoietic stem and progenitor cells (HSPCs) induced directly by cytotoxic therapies has been challenged by newer data. Cells carrying leukemic mutations can often be found in the blood of healthy individuals, a condition known as clonal hematopoiesis (CH). These mutant clones can be detected prior to the development of any hematologic malignancy, expand following exposure to chemotherapy, and can evolve into tMNs. However, much remains unknown about how specific therapy exposures shape the evolution and somatic mutation profiles of CH and how this complex interplay leads to the development of overt leukemia.
Understanding how hematopoietic cells evolve under the selective pressures of therapy is a major focus of the Sperling lab. A better understanding of how and under what circumstances, CH evolves to tMN may provide us an opportunity to intervene early to prevent disease development.
Publications
- Lenaliomide promotes the development of TP53-muated therapy-related myeloid neoplasms. Sperling AS*, Guerra VA*, Kennedy JA*, Yan Y*, Hsu JI, Wang F, Nguyen AT, Miller PG, McConkey ME, Quevedo Barrios VA, Furudate K, Zhang L, Kanagal-Shamanna R, Zhang J, Little LD, Gumbs CE, Daver NG, DiNardo CD, Kadia TM, Ravandi F, Kantarjian HM, Garcia-Manero G, Futreal A, Ebert BL*, Takahashi K*. Blood. 2022 Oct 20;140(16):1753-63. doi: 10.1182/blood.2021014956.
- Clonal hematopoiesis is associated with increased risk of progression of asymptomatic Waldenström macroglobulinemia. Tahri S, Mouhieddine TH, Redd RA, Lampe LM, Nilsson KI, El-Khoury H, Su NK, Nassar AH, Adib E, Bindra G, Abou Alaiwi S, Trippa L, Steensma DP, Castillo J, Treon SP, Ghobrial IM, Sperling AS. Blood Advances. 2022 Apr 12;6(7):2230-5. doi: 10.1182/bloodadvances.2021004926.
- Association of Clonal Hematopoiesis with Chronic Obstructive Pulmonary Disease. Miller P, Qiao D, Rojas-Quintero J, Honigberg MC, Sperling AS, Gibson CJ, Bick AG, Niroula A, McConkey ME, Sandoval B, Miller B, Shi W, Viswanathan K, Leventhal MJ, Werner L, Moll M, Cade B, Barr RG, Correa A, Cupples LA, Gharib SA, Jain D, Gogarten S, Lange L, London S, Manichaikul A, O’Connor G, Oelsner E, Redline S, Rich SS, Rotter JI, Ramachandran V, Yu B, Sholl LM, Neuberg D, Jaiswal S, Levy B, Owen C, Natarajan P, Silverman EK, van Galen P, Tesfaigzi Y, Cho M, Ebert BL. Blood. 2022 Jan 20;139(3):357-68. doi: 10.1182/blood.2021013531.
- Clonal hematopoiesis in patients receiving chimeric antigen receptor T-cell therapy. Miller PG*, Sperling AS*, Brea EJ, Leick MB, Fell GG, Jan M, Gohil SH, Tai YT, Munshi NC, Wu CJ, Neuberg DS, Maus MV, Jacobson C, Gibson CJ*, Ebert BL*. Blood Adv. 2021;5(15):2982-6. doi: 10.1182/bloodadvances.2021004554.
- Contribution of Clonal Hematopoiesis to Adult-Onset Hemophagocytic Lymphohistiocytosis. Miller PG, Sperling AS, Gibson CJ, Viswanathan K, Castellano CA, McConkey ME, Ceremsak JJ, Taylor MS, Birndt S, Perner F, Arnason JE, Agrawal M, Schram A, Nikiforow S, Pihan G, Hasserjian RP, Aster JC, La Rosée P, Morgan EA, Berliner N, Ebert BL. Blood. 2020 Dec 24;136(26):3051-5. doi: 10.1182/blood.2020008206.
- Fitness Landscape of Clonal Hematopoiesis Under Selective Pressure of Immune Checkpoint Blockade. Miller PG, Gibson CJ, Mehta A, Sperling AS, Frederick DT, Manos MP, Miao B, Hacohen N, Hodi FS, Boland GM, Ebert BL. JCO Precis Oncol. 2020;4:PO.20.00186. doi: 10.1200/PO.20.00186.
- Clonal hematopoiesis is associated with adverse outcomes in multiple myeloma patients undergoing transplant. Mouhieddine TH, Sperling AS, Redd R, Park J, Leventhal M, Gibson CJ, Manier S, Nassar AH, Capelletti M, Huynh D, Bustoros M, Sklavenitis-Pistofidis R, Tahri S, Hornburg K, Dumke H, Itani MM, Boehner CJ, Liu CJ, AlDubayan SH, Reardon B, Van Allen EM, Keats JJ, Stewart C, Mehr S, Auclair D, Schlossman RL, Munshi NC, Anderson KC, Steensma DP, Laubach JP, Richardson PG, Ritz J, Ebert BL, Soiffer RJ, Trippa L, Getz G, Neuberg DS, Ghobrial IM. Nat Commun. 2020 Jun 12;11(1):2996.
- Myelodysplastic syndromes (MDS) occurring in Agent Orange exposed individuals carry a mutational spectrum similar to that of de novo MDS. Sperling AS, Leventhal M, Gibson CJ, Ebert BL and Steensma DP. Leuk Lymphoma. 2020;61(3):728-731.
The genetics of myelodysplastic syndrome: from clonal haematopoiesis to secondary leukaemia. Sperling AS, Gibson CJ and Ebert BL. Nat Rev Cancer. doi: 10.1038/nrc.2016.112. 2016.